Abstract
Introduction: Lenalidomide (Len) in association with standard Rituximab-CHOP (R2CHOP21) has been shown to be safe and effective in newly diagnosed Diffuse Large B-cell Lymphomas (DLBCL) [Nowakowski et al. JCO 2014, Vitolo et al. Lancet Oncol 2014]. The addition of lenalidomide appears to benefit primarily patients with non-Germinal Center B-cell (non-GCB) phenotype as determined by immunohistochemistry (IHC). These early results led to currently ongoing randomized trial in ABC subtype of DLBCL, however efficacy of R2CHOP in ABC DLBCL as defined by Gene Expression Prophyling (GEP) has not been reported. In the present combined analysis of two independent phase 2 studies, we report the long-term follow-up (FU) outcome in DLBCL patients treated with R2CHOP, comparing GCB and ABC according to Nanostring Platform.
Methods: We included all newly diagnosed histologically-confirmed de-novo DLBCL patients enrolled in two R2CHOP21 phase 2 trials, conducted by Mayo Clinic (MC) and Italian Lymphoma Foundation (FIL). Inclusion criteria in the two trials were similar, main differences included: all age and all International Prognostic Index (IPI) vs age between 60 and 80 years old and IPI >1, in MC vs FIL study, respectively. All pts received R-CHOP21 plus Len at 25 mg/day for 10 days/cycle and 15 mg/day for 14 days/cycle in MC and FIL trial, respectively. Cell of origin (COO) was determined by IHC, according to Hans algorithm, and retrospectively, in patients with available pathological material, by Nanostring, performed according to Scott algorithm and Masque-Soler signature in MC and FIL study, respectively. We analyzed the long-term FU outcome in terms of progression-free survival (PFS), time to progression (TTP), overall survival (OS), comparing between GCB and ABC phenotypes according to GEP.
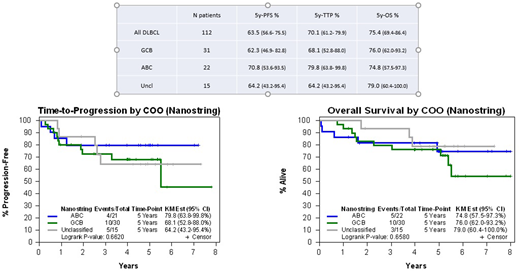
Results: A total of 112 DLBCL pts (63 MC, 49 FIL) were included. Main characteristics were: median age 69 years (y) (range 22-87), male 65 (58%) pts, advanced stage III-IV in 94 (84%), B symptoms in 38 (34%), International Prognostic Index (IPI) intermediate-high/high in 71 (63%). At a median follow-up of 5.1 years (y), 5y-PFS was 63.5%, 5y-TTP 70.1% and 5y-OS 75.4%. A total of 32 relapses were observed, with only 2 cases of Central Nervous System (CNS) relapse. Late relapse occurring beyond 3 years was observed in 4 cases (3 cases with GCB phenotype and one case with missing COO data). In a subgroup analysis by IPI 0-2 vs 3-5 were: 5y-PFS, 5y-TTP and 5y-OS were: 69.0% vs 59.0% (p=0.100), 73.2% vs 67.4% (p=0.285) and 82.3% vs 70.2% (p=0.059), respectively. Regarding for COO defined by IHC, GCB phenotype vs non-GCB were 45 (40%) vs 41 (37%) patients respectively; 26 (23%) patients were not evaluable. 5y-PFS, 5y-TTP and 5y-OS were: 52.8% vs 64.5% (p=0.198), 61.6% vs 69.6% (p=0.444) and 68.6% vs 74.1% (p=0.238) in GCB vs non-GCB, defined by IHC, respectively. Regarding Nanostring analysis, GCB vs ABC vs Unclassified (Uncl) were 31 (46%) vs 22 (32%) vs 15 (22%) respectively; 44 pts were not evaluable. 5y-PFS, 5y-TTP and 5y-OS were: 62.3% vs 70.8% vs 64.2% (p=0.645), 68.1% vs 79.8% vs 64.2% (p=0.662) and 76.0% vs 74.8% vs 79.0% (p=0.658) in GCB vs ABC vs Uncl, defined by Nanostring, respectively (Table1, Fig1).
Conclusions: The association of Len with R-CHOP21 appears to overcome the negative prognostic impact of ABC phenotype determined by GEP, with high PFS, TTP and OS rate, maintained at a long-term FU analysis.
Chiappella:Teva: Other: lecture fees; Janssen: Membership on an entity's Board of Directors or advisory committees, Other: lecture fees; Nanostring: Other: lecture fees; Roche: Other: lecture fees; Amgen: Other: lecture fees; Celgene: Membership on an entity's Board of Directors or advisory committees, Other: lecture fees. Gaidano:Roche: Consultancy, Honoraria; Amgen: Consultancy, Honoraria; Janssen: Consultancy, Honoraria; Gilead: Consultancy, Honoraria; AbbVie: Consultancy, Honoraria; Morphosys: Honoraria. Witzig:Celgene: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding. Vitolo:Janssen: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Celgene: Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Takeda: Speakers Bureau; Roche: Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Sandoz: Speakers Bureau; Gilead: Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal